Hepatitis C Project
Project name: Improving screening and treatment for patients at-risk for Hepatitis C through an embedded clinical pharmacist
Principal Investigator: Amber Slevin, PharmD, BCACP, CTTS
Dr. Slevin is an accomplished clinical pharmacist, with significant post-graduate experience in Hepatitis C treatment.
Timeline: October 2019 to September 2020
Funding: North Dakota Department of Health
Collaborators: Family HealthCare, North Dakota Department of Health, NDSU
Purpose:
The North Dakota Department of Health wanted to improve screening of patients at-risk for Hepatitis C at a Federally Qualified Health Center (Family HealthCare, Fargo, ND) in order to optimize linkage to treatment, to increase the number of patients receiving treatment with direct acting agents, and to increase treatment success.
What is already known on this topic?
Hepatitis C virus (HCV) is prevalent among and transmitted by people who inject drugs (PWID). The current rates of HCV screening and treatment are insufficient to affect population-wide HCV outcomes, particularly among PWID.
What is the impact of this project on collaboration and advancement in pharmacy?
It has shown the essential role that clinical pharmacists play in Hepatitis C screening and treatment. It has also shown the importance of integrating pharmacy practice into public health interventions funded by the state health department.
What is the impact of this project on improving population health?
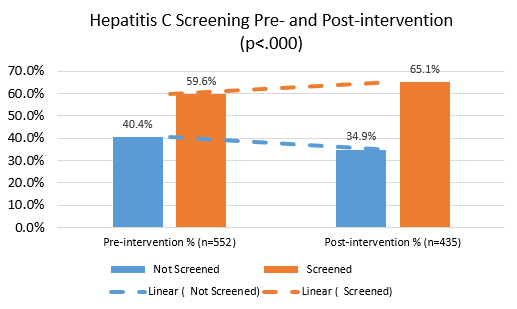
This project analyzed a multifaceted intervention in a federally qualified health center that resulted in a modest increase in the proportion of PWID screened for HCV. We also found possible barriers to optimizing HCV care.
This project has also shown some of the challenges that exist with risk-based HCV screening. While universal screening guidelines implemented in 2021 may overcome some of these barriers, additional efforts are needed to diagnose and ultimately treat HCV among people who inject drugs to reduce population-wide prevalence and transmission.
What publications, presentations, posters, and follow up grants did this work lead to?
Peer-reviewed publications
Perkins M, Slevin A, Strand MA, Freisner D. Screening for Hepatitis C in Persons Who Inject Drugs: A Public Health Crisis Calling for Improvement. Accepted to Preventing Chronic Disease, March, 2021. ( https://www.cdc.gov/pcd/issues/2020/pdf/20_0350.pdf )
https://www.cdc.gov/pcd/issues/2020/pdf/20_0350.pdf )
Poster presentations
Kessel K, Perkins M, Slevin A. “People who inject drugs identified for hepatitis C virus screening: does documentation in the problem list solve all problems?” Student poster presentation at American Society of Health-System Pharmacists Midyear Clinical Meeting (Virtual), December 2020.
Rummel K, Maack B, Slevin A, Strand M. “Implementation of a hepatitis c virus (HCV) screening process within a multisite clinic system by ambulatory care pharmacists.” Student poster presentation at American Society of Health-System Pharmacists Midyear Clinical Meeting (Virtual), December 2020.
Slevin A, Maack B, Strand M, Perkins M, Kessel K, Friesner D. “Threading the needle: a quality improvement project to optimize intravenous drug use documentation and subsequent healthcare.” Poster presentation at American College of Clinical Pharmacists Annual Meeting (Virtual), October 2020.
Perkins M, Slevin A, Klapperich A. “Intravenous drug use documentation: finding a needle in a haystack.” Poster presentation at American Society of Health-System Pharmacists Midyear Clinical Meeting, Las Vegas, Nevada; December 2019.
Bergh T, Slevin A, Dwyer T. “Screenplay: the local story of baby boomers and hepatitis C virus screening.” Poster presentation at American Society of Health-System Pharmacists Midyear Clinical Meeting, Las Vegas, Nevada; December 2019.
Podium presentations
Slevin A and Renton S. “Hepatitis C Treatment in the Primary Care Setting: Increasing Access to Curative Therapies.” Dakota Conference on Rural and Public Health, Virtual, June 3, 2021.


