Estelle Leclerc, PhD

Associate Professor
5 Sudro Hall
Office Phone 701.231.5187
Lab Phone 701.231.5564
Estelle.Leclerc@ndsu.edu
Education
1994 PhD, University Paris XI, France
Positions
1994 - 1998 Post-doctoral Associate, ETH-Zürich, Switzerland
1998 - 2003 Post-doctoral Associate, The Scripps Research Institute, La Jolla, CA
2004 Junior Group Leader, Children’s Hospital, Zürich, Switzerland
2005 - 2009 Research Assistant Professor, Florida Atlantic University, Boca Raton, FL
Fall 2009 Assistant
Professor of Pharmaceutical Sciences, NDSU, Fargo, ND
Research Interests
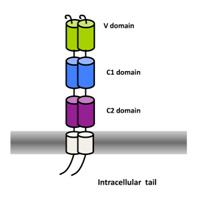
The Receptor for Advanced Glycation Endproducts (RAGE) is an immunoglobulin like receptor involved in major human diseases such as cancer, diabetes or Alzheimer’s Disease. In several mouse models of human pathologies or diseases, antibodies against RAGE have shown promising results.
My laboratory is interested in developing new monoclonal antibodies that can block the interaction of RAGE with its ligands. In the laboratory, we use methods of Molecular Biology, Biochemistry and Cell Biology.
Recent Publications
Ostendorp, T., Weibel, M., Leclerc, E., Kleinert, P., Kroneck, P.M.H., Heizmann, C.W. and Fritz, G. (2006), Biochem. Biophys. Res. Comm. 347, 4-11, Expression and purification of the soluble isoform of human receptor for advanced glycation end products (sRAGE) from Pichia pastoris.
Dattilo, B.M., Fritz, G., Leclerc, E., Kooi, C.W., Heizmann, C.W. and Chazin, W.J. (2007), Biochemistry 46, 6957-6970, The extracellular region of the receptor for advanced glycation end products is composed of two independent structural units.
Leclerc, E., Fritz, G., Weibel, M, Heizmann, C.W. and Galichet, A. (2007), J. Biol. Chem. 282, 31317-31331, S100B and S100A6 differentially modulate cell survival by interacting with distinct RAGE immunoglobulin domains.
Ostendorp, T., Leclerc, E., Galichet, A., Koch, M., Demling, N., Weigle, B., Heizmann, C.W., Kronek, P.M. and Fritz, G. (2007), EMBO J. 26, 3868-78, Structural and functional insights into RAGE activation by multimeric S100B.
Buetler, T.M., Leclerc, E., Baumeyer, A., Latado, H., Newell, J., Adolfsson, O., Parisod, V., Richoz, J., Maurer, S., Foata, F., Piguet, D., Junod, S., Heizmann, C.W. and Delatour, T. (2008), Molecular Nutrition and Food Research 52, 370-378. N-carboxymethyllysine-modified proteins are unable to bind to RAGE and activate an inflammatory response.
Sturchler, E., Galichet, A., Weibel, M., Leclerc, E. and Heizmann, C.W. (2008), J. of Neuroscience. 28(20):5149-5158. Site Specific Blockade of RAGE-Vd prevents amyloid-beta oligomers neurotoxicity.
Leclerc, E., Fritz, G., Vetter, S. and Heizmann, C.W. (2008), B.B.A. M.C.R., 1793:993-1007 Interaction of S100 proteins with RAGE: an update.
Leclerc, E., Sturchler, E. and Heizmann, C.W. (2009) Book chapter in “Handbook of Neurochemistry and Molecular Neurobiology”, K. Mikoshiba Ed., Springer Publishers, New York. Vol. 1, Chapt. 27, 509-532. Calcium Regulation by EF-hand Proteins in the Brain.
Leclerc, E., Sturchler, E., Vetter, SV. and Heizmann, C.W. (2009), Reviews in the Neurosciences 20: 95-110. Cross-talk between Calcium, amyloid and the Receptor for Advanced Glycation Endproducts in Alzheimer’s Disease.
Leclerc, E., Sturchler, E. and Vetter, S. W. (2010), Cardiovascular Psychiatry and Neurology 539581 (DOI:10.1155/2010/539581). The S100B/RAGE Axis in Alzheimer’s Disease.
Leclerc, E. and Heizmann, C.W. (2011) Reviews in Biosciences, S3: 1232-1262. The importance of Ca2+/Zn2+ signaling S100 proteins and RAGE in translational medicine.
Leclerc, E. (2011) Book chapter in “Breakthroughs in Melanoma Research”, Tanaka Y. Ed. InTech Publishers, Rijeka, Croatia. Vol.1: 331-356.
Buetler, T.M., Latado, H., Leclerc, E., Weigle, B., Baumeyer, A., Heizmann, C.W. and Scholz, G. (2011), Molecular Nutrition and Food Research, 55: 291-299. Glycolaldehyde-modified beta-lactoglobulin AGEs are unable to stimulate inflammatory signaling pathways in RAGE-expressing human cell lines.


