An Investigation of Ozone Consumption Rates and By-Product Formation at the Moorhead Water Treatment Plant

Stuart Hurley. Stuart received a B.S. in Civil Engineering from NDSU, and is currently pursuing a master’s degree in Environmental Engineering, also at NDSU. Part of his research time is spent at the Moorhead Water Treatment Plant. Upon graduation he intends to work for an environmental engineering firm or government agency in the area of water quality. Stuart plans to complete course requirements and thesis research in 2001.
stuart_hurley@hotmail.com
Fellow: Stuart Hurley, Environmental Engineering Program, Department of Civil Engineering, NDSU
Advisor: Wei Lin, Assistant Professor of Civil Engineering, NDSU
Matching Support: Moorhead Water Treatment Plant; Fargo Water Treatment Plant
Degree Progress: M.S. expected in 2001.
An Investigation of Ozone Consumption Rates and By-Product Formation at the Moorhead Water Treatment Plant
The Moorhead Water Treatment Plant employs ozone for the purposes of disinfection and removal of taste and odor compounds. Ozone is a powerful oxidant that either completely oxidizes organic compounds into carbon dioxide and water, or partially oxidizes organics into simpler compounds also known as disinfection by-products. Some disinfection by products are of concern as drinking water pollutants.
The raw water or influent to the Moorhead water treatment facility consists of both well water and Red River water. The first stage of treatment is lime and soda ash softening which raises the pH of the water from about 8 to around 10.6-11.3. The water is then sent through the ozone contact chamber for primary disinfection. The ozone contact chamber consists of six cells. In the first cell the water is ozonated without any pH adjustment from the softening basins for oxidation of taste and odor compounds. In the third cell the water is ozonated and recarbonated with carbon dioxide to reduce the pH to around 9.5. In the fifth and sixth cells the water is ozonated again to meet disinfection requirements. From the ozone contact chamber the water is filtered then a chloramine secondary disinfectant is added to provide residual protection for the distribution system.
The purposes of this project are to identify as many of the DBPs produced by ozone oxidation as possible, to determine the operational conditions associated with the formation of these DBPs, and to investigate operational alternatives to improve ozonation efficiency.
A series of process sampling events and sample analyses were carried out to determine the extent of organic compound oxidation and ozonation by-product formation. Chemical oxygen demand (COD) and total organic carbon (TOC) were analyzed to determine the amount of oxidation and partial occurring across the ozone contact chamber. Decreased concentrations of TOC indicate complete oxidation of organic matter to inorganic products, while a COD reduction can be resulted from partial and complete oxidation of organic compounds. The results of this analysis showed a 10-35% COD reduction and generally less than a 10% TOC removal occurring in the ozone contact chamber indicating partial oxidation was taking place.
Two gas chromatograph mass spectrometer (GC/MS) procedures were developed to study the formation of ozonation by-products. The majority of ozone DBPs are oxygenated and polar in nature such as carbonyl compounds like aldehydes and ketones. An EPA purge and trap procedure was employed to identify volatile carbonyl compounds and another EPA derivatization procedure was used to quantify the carbonyl compounds produced through ozone oxidation.
Water samples have been taken and analyzed three times a week to quantify the disinfection by-products (DBPs) produced through ozone oxidation. Samples were drawn from (1) the ozone chamber influent, (2) after initial ozonation at high pH, (3) after recarbonation and pH adjustment, (4) after final ozonation for disinfection, and (5) after filtration. The first four sample points will provide information on what is occurring across the ozone contact chamber, and the filter effluent data will be used to assess the performance of the filters on removal of the DBPs as a result of biological oxidation occurring in the thin biofilm attached to the granular filter media. The study will continue through December to identify the effects of seasonal changes on the ozonation system as well as the types and concentration of DBPs produced.
The volatile analysis did not provide any conclusive evidence of volatile compounds produced through the ozonation process. There were very few compounds detected, and these were not produced on a consistent basis. The analytes that may be quantified by the carbonyl compound derivatization procedure are formaldehyde, acetaldehyde, propanal, butanal, crotonaldehyde, pentanal, hexanal, cyclohexanone, heptanal, octanal, benzaldehyde, nonanal, decanal, glyoxal, and methyl glyoxal. The major carbonyl compounds identified thus far are formaldehyde, acetaldehyde, butanal, glyoxal, and methyl glyoxal. The concentrations of these five compounds were summed together and are shown in Figure 1 for the ozone contact chamber influent, effluent and the subsequent filter effluent.
Figure 1. 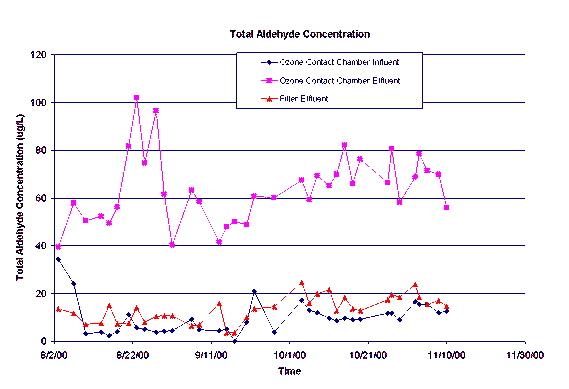
The total aldehyde concentration of the ozone contact chamber effluent is higher than the influent to the contact chamber indicating the formation of carbonyl compounds due to ozone oxidation. An important factor in the treatment process is the removal of these compounds. The filter effluent data provided in Figure 1 indicates the subsequent removal of the majority of these compounds before they actually reach the distribution system for domestic use. The removal of the aldehydes in the filters indicates that the filters are biologically active during this phase of the study.


Wei Lin
Civil & Environmental Eng.
Office: Civil/Ind Eng 201D
Telephone: 701-231-6288
Email: wei.lin@ndsu.edu


