Application of Green Iron Nanoparticles Synthesized Using Barley Polyphenols to Combat

Hoang Nu Kim Pham This is my first year pursuing a PhD degree in Environmental and Conservation Sciences at North Dakota State University. I have a bachelor’s degree in Biology from the University of Dalat (Vietnam) and a master’s degree in environmental science from the University of Newcastle (Australia). I worked at Tay Nguyen Institute for Scientific Research as a microbiology researcher from 2000 to 2015. My work was to produce microbial products that were to be applied in agriculture in the city to reduce the impacts of chemical fertilizers and pesticides on the environment. I also worked projects on evaluating the biodiversity fungi and microorganisms in Dalat city (Vietnam), and make a collection of soil fungi and mushrooms.
Fellow: Hoang Nu Kim Pham
Adviser: Achintya Bezbaruah
Application of Green Iron Nanoparticles Synthesized Using Barley Polyphenols to Combat
Nutrient problem that is caused by the excess of nutrients such as phosphorous and nitrogen in the aquatic environment is a widespread national problem. In North Dakota, two rivers, including the Red River of the North flowing to Lake Winnipeg and the Missouri River joining to the Mississippi River, are also affected by the nutrient pollution. A report of the North Dakota Department of Health indicated that some of 51 river and stream segments, which were listed for biological impairments, and 42 lakes and reservoirs have been assessed as threatened because of nutrients in North Dakota currently (North Dakota Department of Health, 2016).
Besides the natural factors, many other different human activities also cause an increase in loading rates of the two primary nitrogen and phosphorus compounds affecting to North Dakota’s lakes, reservoirs, rivers, and streams (North Dakota Department of Health, 2016).
Phosphorus is one of the key elements necessary for the growth of plants and animals. In the environment, phosphorus exists under the form of phosphates PO4. Phosphates enter waterways from human and animal waste, phosphorus rich bedrock, laundry, cleaning, industrial effluents, and fertilizer runoff. These phosphates become harmful when they over fertilize aquatic plants and cause stepped up eutrophication.
Because the excess of phosphorus in water affects on aquatic life, various techniques have been employed to remove phosphate from water. However, these methods are not much effective in the case of low concentration (several mg/L) of phosphates (Markebs et al., 2016). It is necessary to find a highly efficient and low-cost process to remove phosphorus. 19 Over the last two decades, the application of nanotechnology to remediate contaminants in water has attracted many studies and continues to develop. Studies have shown that iron nanoparticles (Fe-NPs) are very effective for the degradation of halogenated solvents. Besides, iron nanoparticles are also shown to be effective against some pesticides, heavy metals, and dyes.
However, there is growing concern in the biological and environmental safety of NPs synthesized from physical and chemical synthesis approaches such as high cost of manufacturing, the usage of toxic chemicals, formation of hazardous byproducts, and contamination from precursor chemicals. Therefore, the application an advanced method, which is more stable, degradable, non-toxic, less time consuming and environmental friendly is necessary.
Until now, Fe-NPs have mostly synthesized using different plant extracts. Polyphenols ingredients in plants act as reducing/stabilizing agents that are degradable and low cost. The most common used and intensively studied for the synthesis of Fe-NPs is tea extract. Fe-NPs were synthesized by Hoag et al. by using green tea (Camellia sinensis) extract to react with 0.1 M FeCl3 solution. Other papers use different tea extracts and/or different precursor to synthesize Fe-NPs. The polyphenols of these extracts served as the reducing/capping agents (Herlekar et al., 2014)
Barley (Hordeum vulgare L.) is the oldest domesticated grain that has been cultivated for at least 10,000 years. Two grades of barley are produced in North Dakota. The lower grade is used for high quality livestock feed for dairy and beef cattle and pigs. The higher grade malting barley is for human consumption, mainly for brewing beer. The barley is processed into malt, the same ingredient used in malted milk shakes. A 48-pound bushel of barley will produce about 525 12-ounce bottles of beer. Pearled or hulled barley is an increasingly popular ingredient in cereals, soups, salads and desserts. North Dakota leads the nation in barley production corn (ND Department of Agriculture, 2016).
Significance of Research and Findings
Research Task 1: Barley extract preparation:
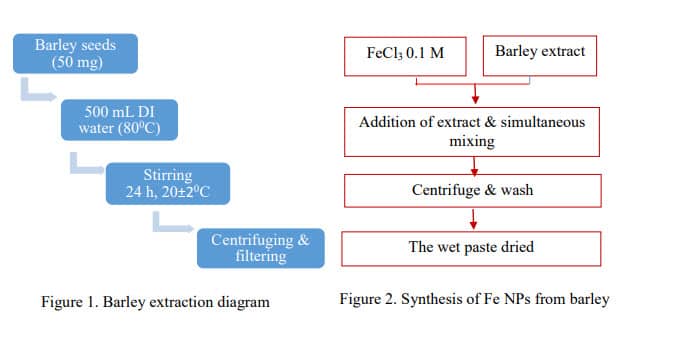
Barley seeds (tradition variety) were provided by the Department of Plant Pathology (NDSU). Fifty grams of finely ground barley samples were extracted twice with 500 mL of water and lightly stirred (24 h, room temperature 22±2oC). The supernatants were collected after being centrifuged (3200 rpm, 15 min, room temperature). pH of the extraction ranges in 5.5 to 6.0. The extract is stored at -4 0C for further use (Figure 1).
Research Task 2: Synthesis of iron nanoparticles:
The synthesis was carried out by adding the corresponding extracts to 0.1 M FeCl3 (pH = 1.60) with the ratio of 1:1 at room temperature, adjusting pH until 6 by the solution NaOH 2M, and constantly stirring for 12 hours. The as-prepared Fe nanoparticles, then, were 20 centrifuged and washed thoroughly three times with deionized (DI) water, last time with ethanol 70%. They were dried under vacuum for 12 h. Barley extract-based Fe-NPs were ground to powdery form and kept under nitrogen gas for further use (Figure 2).
Research Task 3: Characterizations:
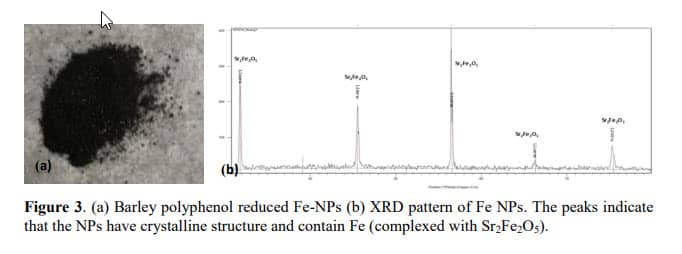
The X-ray diffraction (XRD) was used to analyze the crystallinity of the barley extract-based Fe nanoparticles. The samples were placed in stainless steel sample holders and XRD patterns were recorded using Cu Ka radiation (k = 1.5418 A˚) on a Philips X’Pert diffractometer operating at 40 kV and 40 mA between 5 and 90 (2h) at a step size of 0.0167.
Figure 3 shows the XRD pattern of synthesized iron nanoparticles. On the XRD picture, there are five peaks and all together these peaks are characteristic peaks for compound called Strontium Iron Oxide. Meanwhile, there is no peak characterizing for regular crystal as seen in other plant-based Fe NPs.
Task 4: Batch experiments:
Phosphate removal batch study: the sorption capacities of Fe-NPs were evaluated. Fe-NPs (20 mg in 50 mL or 400 mg/L) were added into phosphate solution (50 ml of 5 mg PO43- - P/L) in multiple 50 mL polypropylene plastic vials fitted with plastic caps (reactors). The reactors were rotated end-over-end at 28 rpm in a custom-made shaker to reduce mass transfer resistance. Three of the reactors were withdrawn at specific time interval (0, 10, 20, 30, 60 min) and the content in reactor was centrifuged at 4,000 rpm (Almeelbi & Bezbaruah, 2012).
Similar experiments were carried out with NZVI (Figure 6 and 7) and green tea extract reduced Fe-NPs (Figures 8 and 9). The bulk solution was analyzed for phosphate concentration using a UV-vis Spectrophotometer. Ascorbic acid method was used for phosphate analysis. This method depends on the formation phosphomolybdic acid during the reaction between orthophosphate and molybdate. Ascorbic acid reduces phosphomolybdic to form a blue complex. The color was measured in the UV-Vis Spectrophotometer (HACH, DR 5000) at wavelength of 880 nm. A five-point calibration was done routinely.
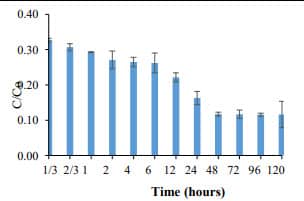 Figure 4. Phosphate removal by barley extract-based Fe NPs (400 mg/L) (C0=5 mg PO43- -P /L). 88.2% phosphate removal was achieved and unchanged after 48 h | 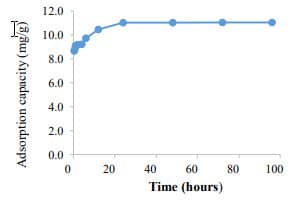 Figure 5. Adsorption capacity of barley extract-based Fe NPs. The adsorption capacity was found to 11.55 mg PO43- -P /g of Fe-NPs. |
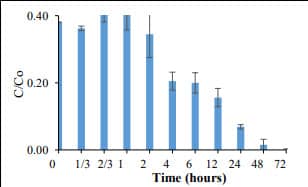 Figure 6. Phosphate removal by conventional Fe NPs (400 mg/L) (C0= 5 mg PO43--P /L). 100% phosphate removal was achieved with 72 h. | 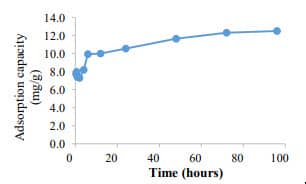 Figure 7. Adsorption capacity of conventional Fe NPs. The adsorption capacity was found to 12.5 mg PO43--P /g of Fe-NPs. |
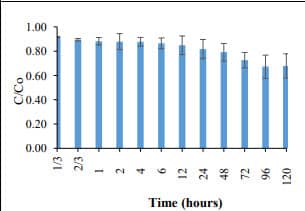 Figure 8. Phosphate removal by green tea extract-based Fe NPs (400 mg/L) (C0= 5 mg PO43--P /L). 20.8% phosphate removal was achieved with 48 h. | 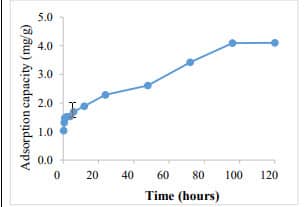 Figure 9. Adsorption capacity of green tea extract-base Fe NPs. The adsorption capacity was found to 4.1 mg PO43- -P /g of Fe-NPs. |
Publication
Pham, H., A. N. Bezbaruah. Biosynthesis of iron nanoparticles using green tea extract for the removal of phosphate from aqueous solution. Journal of Nanoparticle Research, in preparation.
Presentations
Poster Presentation: “Biosynthesis of Iron Nanoparticles Using Green Tea Extract for the Removal of Phosphorus from Aqueous Solution” – 2018 ND Water Quality Monitoring Conference.

Achintya Bezbaruah
Civil & Environmental Eng.
Office: Civil/Ind Eng 201G
Telephone: 701-231-7461
Email: a.bezbaruah@ndsu.edu


