Enhanced removal of Poly- and Perfluoroalkyl substances from water

Pavankumar Challa Sasi (left) (scholarship recipient), a Ph.D. student in Civil Engineering, University of North Dakota, Bachelor of Science in Civil Engineering, Anna University, Chennai, India. Master of Science in Civil Engineering, University of North Dakota.
Fellow: Pavankumar Challa Sasi
Adviser(s): Dr. Feng (Frank) Xiao
Enhanced removal of Poly- and Perfluoroalkyl substances from water
Project Impact:
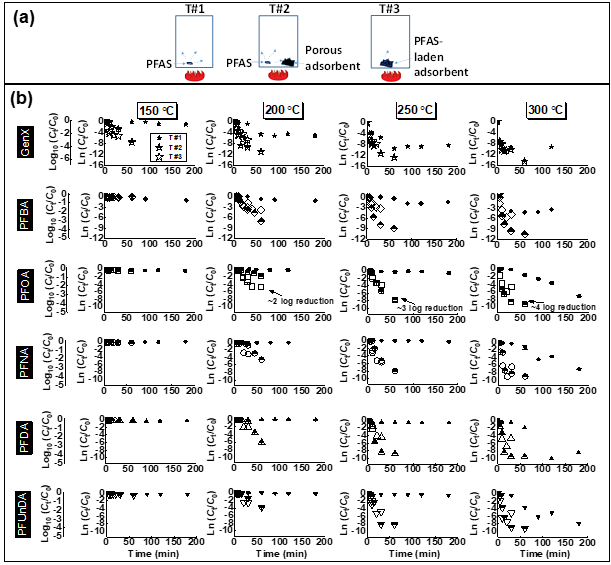
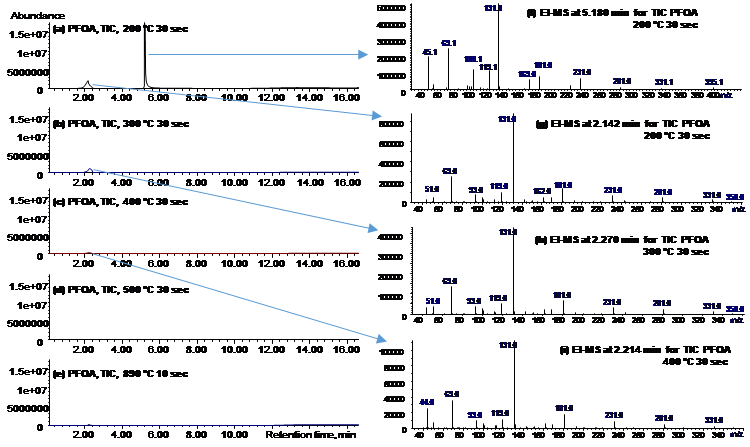
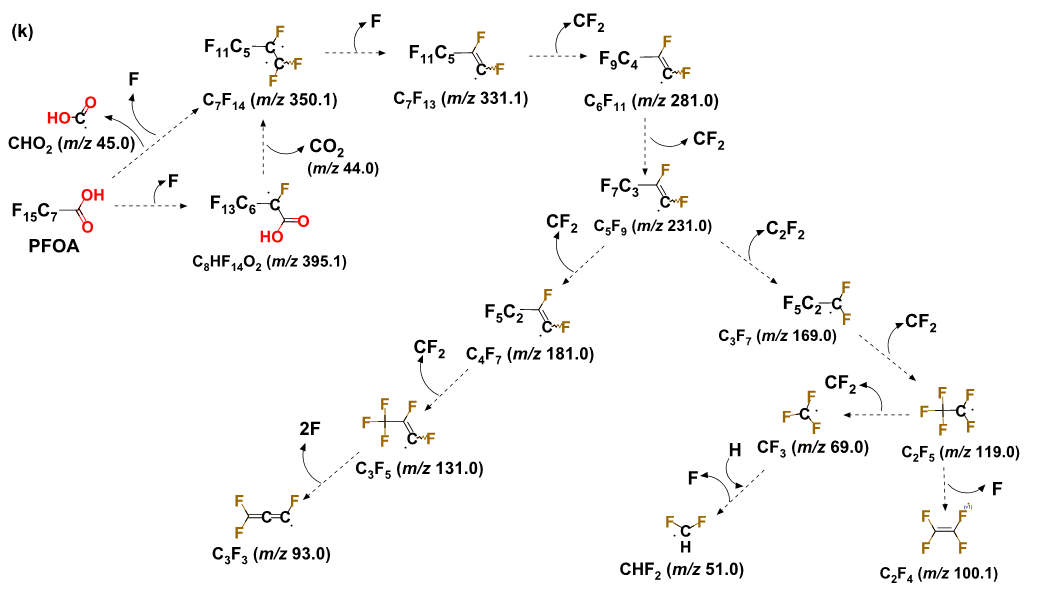
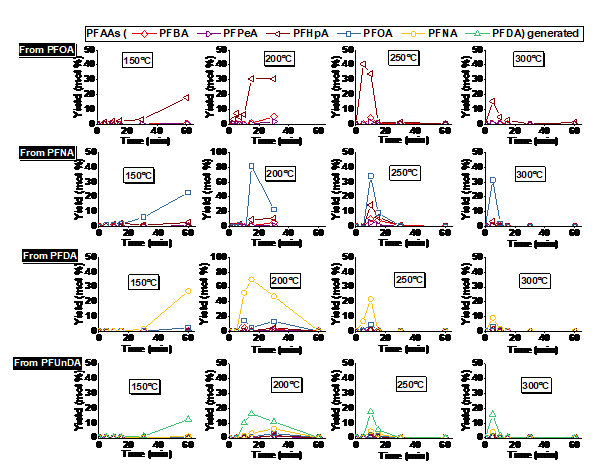
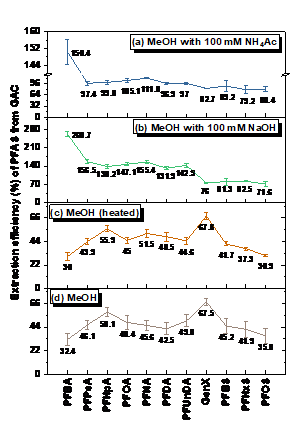
PFAS exhibited the following order of thermal stability: PFSAs >> PFUnDA > PFDA > PFNA > PFOA > PFBA > HFPO-DA. A 30-min thermal treatment of spent GAC in either N2, CO2, or air at ≥300 °C achieved ≥99.9% removal of GenX (HFPO-DA) and ≥99% removal of PFCAs, including PFOA. HFPO-DA was more readily decomposed than PFCA with the same number of perfluorinated carbons(i.e., PFBA), indicating that the perfluorinated chain becomes less thermally stable with the inclusion of an ether group. In continuation to our previous studies and to get a better understanding of PFAS decomposition we studied five perfluoroalkyl carboxylic acids (PFCAs), and one perfluoroalkyl ether carboxylic acid (PFECA) in three different conditions (only PFAS, PFAS with GAC and PFAS adsorbed on GAC) in a closed system. We found that the destabilization of studied compounds during thermal treatment followed first-order kinetics. The temperature needed for thermally destabilizing PFCAs increased with the number of perfluorinated carbons (nCF2) when PFAS were adsorbed on to GAC. Decomposition of PFCAs such as perfluorooctanoic acid (PFOA) on GAC initiated at temperatures as low as 200 °C. The PFECA was even more readily decomposed than PFCA with the same nCF2. The degradation temperature of PFAS decreased with the presence of GAC and on PFAS laden GAC compared to its absence. In addition to the volatile organofluorine species identified in previous studies we found evidence for the formation and then decomposition of short-chain compounds during thermal degradation of four PFCAs. Efficient mineralization to fluoride ions (>80%) of PFOA and PFOS on GAC occurred at 700 °C or higher, accompanied by near complete PFOA and PFOS decomposition (>99.9%). Thermal decomposition pathways of PFOA were proposed. We have also identified that ammonium acetate is the most suitable amendment for methanol to achieve higher PFAS extraction efficiencies. Organofluorine and short-chain compounds generated from thermal decomposition of PFAS at low to moderate temperatures (≤600 °C) warrant studies on the exposure to these compounds during cooking, baking, firefighting, and other relevant thermal processes involving PFAS.
Peer-reviewed Journal Papers
XIAO, F., CHALLA SASI, P., BIN, Y., KUBÁTOVÁ, A. GOLOVKO, S., GOLOVKO, M., DANA, S. “Thermal Stability and Decomposition of Perfluoroalkyl Substances on Spent Granular Activated Carbon” Environmental Science & Technology Letters,https://doi.org/10.1021/acs.estlett.0c00114.

Feng "Frank" Xiao
Civil Engineering
Office: Upson II Room 260K
243 Centennial Drive Stop 8115
Grand Forks, ND 58202-8115
Telephone: 701-777-5150
Email: feng.xiao@UND.edu


